Molecular Gastronomy
How to meet Food and Polymer?
One of the possible answers is the Molecular Gastronomy and I take an example that I love it : the precipitation of sodium alginate into calcium lactate with a liquid inside.
When you click on the following link, you will be able to see a video of the preparation of this molecular reaction
http://www.youtube.com/watch?v=OlJKpt74TvI&feature=fvwrel
Not to let you without any explanation, I am going to give you scientific details about what happens on this video.
First he prepares a aqueous calcium lactate solution from food calcium lactate powder and mixing that he adds to the lemon juice
Then he puts the mixture inside a silicon tin and he freezes the solution in order to solidify it under 0°C
In the meantime, he prepares the sodium alginate solution from food sodium alginate powder and strong mixing, which gives a viscous polymer solution
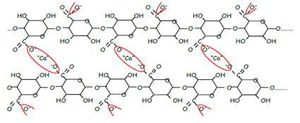
sodium alginate formula
Now he dips the cooled "bubbles" into the sodium alginate and that is the moment when the science works. Indeed thanks to Ca2+ which has 2 charges + the polymer which has only one charge + can react with Ca2+ in order to make a spherical tough covering
Finaly he rinses the bubbles and dips them into different food colorants. All the products used in this videa are safe!
Like that you can make funny little food bubbles for your party which can be made from other liquid like other fruit drink if only the pH stays above 4.
With these little bubbles you are sure to succeed among your friends!
And if you want to impress more your friends, look for any ideas on that link
http://www.moleculargastronomynetwork.com/additives.html





/https%3A%2F%2Fstorage.canalblog.com%2F46%2F55%2F974776%2F74889568_o.jpg)
/https%3A%2F%2Fstorage.canalblog.com%2F21%2F46%2F974776%2F74604748_o.png)
/https%3A%2F%2Fstorage.canalblog.com%2F55%2F98%2F974776%2F74384793_o.jpg)
/https%3A%2F%2Fstorage.canalblog.com%2F79%2F76%2F974776%2F74654075_o.png)